IN VIVO BACKGROUND
With modern genetic techniques allowing scientists to identify the link between genes and diseases, there is an enormous emphasis on determining ways to alter the function of a gene in vivo. Strategies to treat these diseases include delivering an alternate gene to the cell, replacing the defective gene or silencing the defective gene using RNAi or antisense methods.
However, delivery to organs, tissues or cells of therapeutic genetic molecule mimics (DNA, RNA, Protein) is a complex problem, requiring passage across several membranes intended to keep external DNA/RNA out of intercellular compartments. There are viral and non-viral transfection methods for delivering nucleotides to the cell cytoplasm and nucleus. The information on these pages are dedicated so that researchers can find information related to DNA transfection, recombinant plasmid DNA, genetic transformation, a variety of in vivo applications, transfection services and links to commercially available in-vivo transfection reagents.
WHAT DOES “IN VITRO” AND “IN VIVO” MEAN?
When conducting a controlled biological experiment on living cells, there are generally two main categories to classify nearly all experiments. They are named, quite colloquially, “in vitro” and “in vivo“.
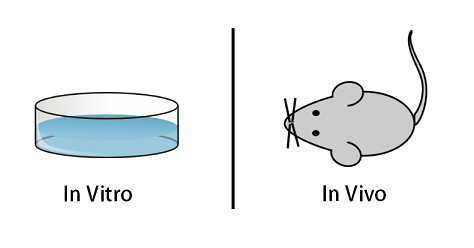
Fundamentally, in vitroincludes experiments conducted in some manner of a container, flask or dish, usually focusing on specific singular types of simple organisms and/or tissues. In vivo includes experiments conducted on living, breathing (in one form or another) multicellular organisms. Both forms of experimentation rely on the study of living cells. It’s important to note that the difference here isn’t life and death, as both the breathing animal and the cells in the petri-dish are both technically alive.
IN VIVO TRANSFECTION CONSIDERATIONS
A transition from in vitro to in vivostudies always brings forth complications not encountered when performing in vitroexperiments. To help guide researchers into the unknowns of in vivo delivery, there are commercially available reagents to side-step common pitfalls such as tissue specificity, efficiency, clearance, half-life, stability of the DNA/RNA mimic, toxicity and immune response. There are many formulations available with these characteristics:
- Lipid-based reagent
- PEG complex
- Nanoparticles
- Polymer-based
IN VIVO TRANSFECTION REAGENTS
Typically, transfection reagents rely on positively charged molecules to achieve gene transfer activity in vitro. However, this positively charged structure poses many problems when injected systemically. Upon injection, positively charged complexes are cleared from circulation. The lack of circulation in the animal results in low bioavailability of the active pharmaceutical ingredient (API) to reach the desired target tissue.
Although highly modified mimics complexed to intense reagent formation can increase bioavailability, the downfall can often be a lack of endosomal release of the payload. An in vivo transfection reagent that can protect from degradation and deliver the API into cells by endocytosis must also have the ability to release the cargo upon delivery. Efficiency, protecting the API, injection route and lack of immune response all need careful consideration in reagent choice.
Commercially available in vivo transfection kits – Link
Biodistribution, PF/PD, xenograft, tox/pharm preclinical research services – Link
IN VIVO TRANSFECTION SAMPLE COLLECTION
Xenograft animal models injected with an in vivo transfection reagent assess the efficacy of drugs against a specific cancer type. Testing novel therapeutics on tumors that have been engrafted by subcutaneous or orthotopic inoculation in an immunocompromised mouse model is the initial steps for all clinically approved anti-cancer drugs. Xenograft studies are complex, requiring the selection of the appropriate animal model, the correct cell line that is tumorigenic, choosing the best drug administration route, dosing schedule, tumor growth rate analysis and quantitation of target genes (i.e. histology, mRNA and protein expression levels). Here are some common xenograft study design considerations and data collection options:
- Dose route (intravenous, intratracheal, continuous infusion, intraperitoneal, intratumoral, oral gavage, topical, intramuscular, subcutaneous, intranasal, using cutting-edge micro-injection techniques and pump-controlled IV injection)
- Dosing frequency and duration
- Tumor immunohistochemistry
- Cell engraftment sites (orthotopic transplantation, tail vein injection and left ventricular injection for metastasis studies, injection into the mammary fat pad, intraperitoneal injection)
- Tumor Growth Delay
- Tumor Growth Inhibition
- Blood chemistry analysis
- Toxicity and survival
- Lipid distribution
- Imaging studies: Fluorescence-based whole body imaging, MRI
At the end of an animal study, all collected tumors and tissues can be snap frozen in liquid nitrogen, submerged in RNAlater and stored indefinitely, the nucleic acids can be isolated for genetic analysis or tissues fixed in formalin for histological analysis.
IN VIVO TRANSFECTION | PEG LIPOSOME TRANSFECTION | NANOPARTICLE TRANSFECTION | LIPID-BASED TRANSFECTION | POLYMER-BASED TRANSFECTION | USES OF DNA TRANSFECTION